What is Neutral Alcohol?
The base of neutral alcohol is ethanol, distilled at a minimum ABV percentage of 95%. Neutral alcohol is also known as a neutral spirit, rectified spirit, rectified alcohol, or ethyl alcohol. In some countries, denatured alcohol or denatured rectified spirit is also known as neutral alcohol. Neutral alcohol can be made from grains, grapes, molasses, potatoes, sugarcane, sugarbeets, and other fermented agricultural products.Ethanol (C2H5OH), also called ethyl alcohol, pure alcohol, or grain alcohol is a volatile, flammable, colorless liquid. It is the principal alcohol found in alcoholic beverages.
Depending on its quality, neutral alcohol is suitable for a variety of applications -
- Neutral alcohol is the base for flavored alcoholic beverages such as blended whiskey, cut brandy, some liquors, and bitters. It is also used to make homemade liquors such as limoncello or cassis.
- It is also used to make vinegar.
- Neutral alcohol is also used in chemical, pharmaceutical, and cosmetic products.
Processing of Neutral alcohol
Neutral alcohol is manufactured through various steps, which are very significant and important.
Collection of raw material
Different raw materials are used to make neutral alcohol, such as grains (barley, wheat, corn, rye, etc.), molasses, potatoes, grapes, sugarcane, sugarbeets, etc. These raw materials are also selected based on suitability to make the final product and availability.Fermentation
At the fermentation, yeast transforms monosaccharides into alcohol and carbon dioxide. The raw materials contain sugar or carbohydrate, which converts to alcohol in the fermentation process. Carbon dioxide is also formed with alcohol. The temperature range for yeast fermentation is in between 90oF - 95oF (32oC - 35oC)
Distillation and Rectification
A small amount of alcohol is produced in the fermentation process, no matter what raw material is used. So the produced alcohol undergoes the distillation process to enhance the concentration.
Two main distillation processes take place: Column distillation and Pot distillation. When the mixture of ethanol and water (a mixture of ethanol and water is called wash) is heated, ethanol boils at a lower temperature (78.5oC) than water (100oC). There are three stages of vaporization -
- Boil-off: In this stage, compounds with the low boiling point vaporizes. It is also called Foreshot or Heads. Generally, the following substances get vaporized during this stage -
- Acetaldehyde (CH3CHO) boils at 20.8oC. It has a pungent fruity odor reminiscent of metallic green apple. It is an aldehyde produced by plants as part of their normal metabolism. Acetaldehyde is also produced by the oxidation of ethanol.
- Acetone ((CH3)2O) is a colorless, flammable liquid with a boiling point of 56.2oC. Acetone is the simplest form of the group ketone. It is commonly used as a cleaning agent.
- Esters (RCO2R/), where R is the hydrocarbon part of the carboxylic acid, R/ is the alcohol, C is the carbon atom and O2 is the oxygen. It is a naturally occurring compound responsible for the aroma of many fruits such as apple, strawberry, banana, etc. Esters include ethyl acetate (boiling point 77.1OC), ethyl butyrate (121OC), ethyl formate (54OC), and hexyl acetate (171.5OC).
- Methanol (CH3OH) boils at 64.7OC. It is also called methyl alcohol. The boiling point of methanol and ethanol is almost the same, so it is very hard to separate them in distillation. However, methanol is separated as it is very bad for the liver, and consumption is likely to lead to blindness.
- The heart or spirit: The heart is the good tasting part of the distillation that is safe to digest. It is the part of a distillate produced during distillation that is separated to make alcoholic beverages. The main substance found in the heart of the distillation is ethanol.
- The tails: Tails are the last part of a distillation process. In this stage, compounds with a high boiling point are found. Those substances are -
- 1-Propanol (CH3CH2CH2OH) is formed naturally during the fermentation process. It has a boiling point of 97OC. It is used as a solvent in the pharmaceutical industry.
- Butyl alcohol or Butanol (C4HO2) has a boiling point of 118OC. It is formed naturally during the fermentation of sugar and other carbohydrates. Butanol has a banana-like flavor.
- Amyl alcohol (C5H12O) is colorless alcohol with a boiling point of 131.6OC. It has a strong smell and sharp burning taste.
- Fusel alcohol, also known as fusel oil includes all the bitter compounds found in the trails during the distillation process. The term fusel is a German word, which means bad liquor. It includes propanol, butanol, and amyl alcohol. Fusels are high order alcohols, have more than two carbon atoms, and significantly soluble in water. It is produced in the fermentation process of various alcoholic beverages.
Distillation methods
A continuous process, where a controlled amount of fermented wash is constantly being pumped into the still, while a steady supply of freshly distilled spirit emerges on the other side. The column is made of several trays or plates. The substance to be distilled (referred to as feedstock) is fed into the column vertically from top to bottom and hot vapors are coming up from bottom to top is a blast of the stream. When the feedstock comes into contact with the stream, it volatilizes ethanol and flavor compounds. As a result, these vapors begin traveling back up through plates. Since hot vapors are constantly coming through the column, the condensed vapors are redistilled and thrown back into a vapor state. As this rapid transformation from vapor to liquid to vapor happens, it separates heavier compounds like fusels from the lighter compounds like ethanol. As a result, it increases the purity of the spirit up to 96% ABV of ethanol.
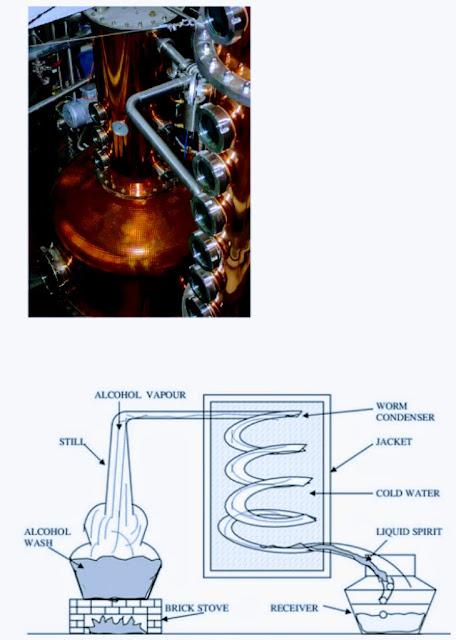
It is the first method in the history of distilling. This distillation is also known as Alembic Distillation as an alembic or a big kettle shaped vessel, where the primary fermented liquid, also known as a wash, is heated up. Ethanol evaporates before the water, traveling into a cooling tube and back into another vessel to condense. Other compounds such as heads and tails (eg. esters, tannins, methanol, fusel alcohols) also evaporate during distilling and can impact the flavor. This distillate produces alcohol, has a concentration of about 20-30% by volume. This lower concentrated alcohol is further distilled a second time to produce more concentrated alcohol.
Different grades of neutral alcohol
Grain Neutral Spirit (GNS)
Neutral spirit or alcohol made from grain is called Grain Neutral Spirit. The most popular grains to make GNS are - barley, corn, wheat, rye, etc. It is distilled up to 95% ABV. Due to the higher level of alcohol GNS is treated with a higher level of care than normal spirit. Some examples of spirits made from GNS are - gin, vodka (ABV - 40%), whiskey (ABV - 40 to 68%), bitters (ABV - 30 to 45%), etc.Potato-based Neutral alcohol
Extra neutral alcohol or potato alcohol is made from potato and is a popular choice to make vodka and aperitifs because it has a slightly creamy-textured which makes it suitable for the base spirit. Potato alcohol is distilled up to 95% ABV. Potato alcohol is gluten-free colorless alcohol with a neutral smell. It is produced in various regions of the Northern Hemisphere such as the US, Canada, Western Europe, and Eastern Europe. Examples - vodkaGrape-based neutral alcohol
Also known as vinous alcohol or wine alcohol, is made from grape residues such as skin, pulp, seed, stem, and the wine lees (sediments dead yeast cells left after fermentation), during wine production. It is distilled up to 96% ABV (sometimes 95.5%). Vinous alcohol is used to make fortified wine such as Sherry, Port wine, etc and also is a key ingredient for making famous Italian grape-based pomace brandy Grappa (ABV - 35 to 60%)Molasses based Extra Neutral Alcohol
Molasses is a final by-product of sugar mill and contains residual sugar, glucose, and fructose. Molasses based ENA is a certified organic product that has a strong aroma which is not preferred by spirit producers especially those making vodka and gin.Sources
https://www.ethimex.com/
https://tipsybartender.com/








No comments:
Post a Comment